
Cause of colour
The colour of a gem diamond is one of the four parameters, that determine the value of a cut and polished stone. Till the present time a completely colourless diamond fetches the highest price. The majority of gem diamonds show not much attractive pale yellow or brown colourings and therewith much reduced value. Coloured diamonds in red, blue or green have been known for long time as relative rarity. But they hardly have been in demand as gem stoners, unless they attracted attention because of exceptional size and intensive colour. For almost longer time there were lovers of stones, who gathered especially charming collections of coloured diamonds. However during the last years developed and widened a higher interest in so called "fancy coloured diamonds". The addition "fancy" describes all coloured diamonds with gem quality except the naturally tinted yellow ("Cape") and brown stones.

If a diamond is coloured, it should absorb determined parts of the visible white light in the wave length range between approx. 400 nm (violet) and 700 nm (red), while the not absorbed residue gives the colour of the stone.
As already mentioned, a clear perfect diamond crystal is highly transparent in the whole visible spectral range, i.e. it is colourless. The cause of colours of diamonds therefore should be defects of the regularity of the carbon atoms in the crystal structure. Such defects can be of chemical nature, when a part of carbon atoms is replaced by other kinds of atoms, or crystal – structural nature, when single carbon atoms or complete atom layers are dislocated by vector quantities in size of the elementary cell. Therefore the colour of diamonds is caused by chemical and crystal – structural defects, often appearing linked with each other. The derivable kinds of defects (approx. 40 for diamonds) can cause absorptions in the whole range of transparency of diamonds (wide bands or smaller lines), but only if they absorb in the visible spectrum we call them colour centres.
Decisive for defects of chemical nature is the nitrogen content, that can be diamonds up to 0,5 %. According to the nitrogen content, which also influences the further physical properties, diamonds are divided into two types.
- Type I with high nitrogen content of approx. between 100 – 3000 ppm (parts per million)
- Type II with low nitrogen content of approx. between 4 – 40 ppm.
Here are some quantified samples for it:
One cubic millimetre (1 mm2) diamond, this corresponds to a brilliant of approx. 1/12 ct. with a rundiste diameter of 3 mm, contains 2.1020 carbon atoms. If we replace one hundred of one million carbon atoms by nitrogen atoms (≡ 100 ppm N), so this one mm3 contains 2.1016 N – atoms. At a content of 100 ppb (parts per billion) N, what is already well under the detection limit of most of the analytical instruments and where the given diamond is considered to be from the nitrogen – free type IIa, it contains still 2.1013 N-atoms/mm3. Theoretically this gives a concentration of 1013/mm3 of colour centres named H3, three neighbouring carbon atoms are replaced by two nitrogen atoms and one vacancy, and an absorption line at a wavelength of 503 nm.
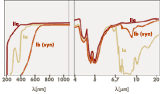 A further subdivision of diamonds follows mainly on the basis of their infrared absorption into Ia (this is with 95 % the largest amount of all diamonds), Ib, IIa and IIb – diamonds. The last-named are extremely rare in nature. Their nitrogen content is very low, so a low boron content cannot be compensated and the boron atoms act as acceptors what makes the diamond a p – type semiconductor. From this follows an optical absorption, which begins in the infrared at about 4 µm and continues into the red range of the spectrum, what can cause an attractive blue colour. A further subdivision of diamonds follows mainly on the basis of their infrared absorption into Ia (this is with 95 % the largest amount of all diamonds), Ib, IIa and IIb – diamonds. The last-named are extremely rare in nature. Their nitrogen content is very low, so a low boron content cannot be compensated and the boron atoms act as acceptors what makes the diamond a p – type semiconductor. From this follows an optical absorption, which begins in the infrared at about 4 µm and continues into the red range of the spectrum, what can cause an attractive blue colour.
At the type Ib – diamonds, which are very rare in nature but nearly all synthetic diamonds are of this type, the nitrogen atoms are isolated as substitutes of carbon atoms in the crystal structure. Nitrogen in this form is often called C. It causes absorption beginning at approx. 500 nm and increases to shorter wavelengths. This causes a pale yellow up to yellowish brown colour depending on concentration and size. In this form causes nitrogen in the crystal paramagnetic resonance.
At the Ia diamonds the nitrogen atoms exist in aggregated form in the crystal structure, i.e. two or more N – atoms as direct neighbours occupy positions of carbon atoms. Pairs of nitrogen atoms are called A-form. If the nitrogen content consists mainly of this form, these diamonds are called IaA – type. It is analogous for the IaB – type, where there are aggregates of four (possible also six or eight) neighbouring nitrogen atoms. Often the nitrogen content exists of approx. The same content of A- and B – forms, then it is called IaAB. A- and B- aggregates don’t cause absorption in the visible region, what seems to be logical, because otherwise there wouldn’t be so many colourless gem diamonds of the Ia type. But the show in the ultraviolet from approx. 320 nm up to the absorption edge of all diamonds at 250 nm an increasing absorption intensity.
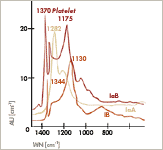 Important for the diagnostics and the analytical derivation of the nitrogen contents are their absorption spectra in the infrared region of the wavelengths between 7 and 10 µm in the so called one – phonon – region. They are formed characteristically as absorption bands with peaks at wave numbers of 1282 cm-1 (A) and 1165 cm-1 (B). An absorption line coupled with often higher B – contents at wave numbers about 1165 cm-1 is caused by so called platelets. While all defects reduced till now to nitrogen and boron are considered to be more or less point defects, we have here extensive defects, aggregates in lamellar form of vacancies and nitrogen atoms, which can have a size of some micrometers and can be then observed by electron microscopy and even light microscopically. Important for the diagnostics and the analytical derivation of the nitrogen contents are their absorption spectra in the infrared region of the wavelengths between 7 and 10 µm in the so called one – phonon – region. They are formed characteristically as absorption bands with peaks at wave numbers of 1282 cm-1 (A) and 1165 cm-1 (B). An absorption line coupled with often higher B – contents at wave numbers about 1165 cm-1 is caused by so called platelets. While all defects reduced till now to nitrogen and boron are considered to be more or less point defects, we have here extensive defects, aggregates in lamellar form of vacancies and nitrogen atoms, which can have a size of some micrometers and can be then observed by electron microscopy and even light microscopically.
At the relative rare IIa diamonds, where less colour centres fixed to nitrogen can be expected, structural defects play a more important role. Here the range covers from point defects (vacancies, interstitials = atoms on interstitial sites) over line defects (dislocation lines of different dislocation types) up to area defects (plastic shear, stacking faults, microcracks)
The brown colouring of the IIa type diamonds with an atypical continuous absorption in the visible region can be mainly reduced to such structural defects, that develop from partly intense plastic deformations. Some discrete absorption lines on both ends of the visible spectrum can be reduced on vacancies.
|


