|
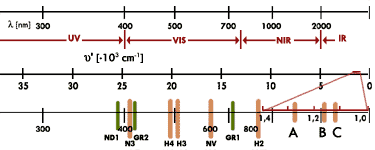
Spectroscopy offers manifold possibilities for the analysis of the wavelength dependent optical properties of materials. With its different methods and instruments it can be used for gem stone diagnostics. and is essential for a detailed investigation of diamonds. Especially interesting for the diamond is the wave length coverage, where it is transparent, so that changes in the transparency or in the absorption behaviour becomes apparent in the spectrum. A chemical clean and crystal - structural undistorted diamond has a high transparency between 225 nm and 2,5 µm as well as above 6 µm (the last named value makes such diamonds interesting as optical windows for infrared in the Cosmos). Therefore spectroscopes should work in the wavelength coverage between approx. 0,2 µm (the adsorption edge in the ultraviolet is at 225 nm) and 20 µm. Because the wavelength λ often is indicated in the inverse and energy proportional dimension of the wave number 1/λ[nm] = ν[cm-1], this corresponds to values of the wave numbers between 50000 cm-1 (≡6,2 eV) and 500 cm-1 (≡ 62 meV). The modern high - resolution and quick - operating, of course in this case mostly quite expensive, instrumental solutions cover in the main two spectral ranges:
- As infrared - spectrometer for the absorption spectroscopy, the Near and Mean InfraRed (NIR - MIR: ν = 7000 - 400 cm-1, λ = 1,4 - 25 µm) and
- For the absorption spectroscopy the Ultraviolet, the Visible region and the Near Infrared (UV - VIS - NIR: λ = 200 - 25 nm, ν = 50000 - 4000 cm-1). The regions also can be dimensioned narrower, what can influence the price. Because the diamonds are small crystals resp. cut stones, the instruments are constructed as microscope spectrometer.
 To 1. The instrument used by us is a FTIR spectrometer, i.e. a Fourier transformed infrared spectrometer. A Fourier transformation, an efficient boundary value solution of a special exponential integral function, is used for the fast and high - resolution analysis. On the basis of the infrared spectra can be made a series of important statements, if the diamond is of type Ia or IIa, how is the relative content of nitrogen - A - and -B - aggregates, i.e. pairs of two nitrogen atoms and tetrahedral arrangement of four nitrogen atoms. From the absorption coefficient of the A- and/or B - absorption bands at ν= 1282 m-1, if necessary under consideration of the absorption portions of isolated nitrogen atoms (C - component) and the platelets (D-, P- or B’ - component), "extensive" defects linked to nitrogen, the nitrogen content can be determined by computational decomposition of the absorption spectra. To 1. The instrument used by us is a FTIR spectrometer, i.e. a Fourier transformed infrared spectrometer. A Fourier transformation, an efficient boundary value solution of a special exponential integral function, is used for the fast and high - resolution analysis. On the basis of the infrared spectra can be made a series of important statements, if the diamond is of type Ia or IIa, how is the relative content of nitrogen - A - and -B - aggregates, i.e. pairs of two nitrogen atoms and tetrahedral arrangement of four nitrogen atoms. From the absorption coefficient of the A- and/or B - absorption bands at ν= 1282 m-1, if necessary under consideration of the absorption portions of isolated nitrogen atoms (C - component) and the platelets (D-, P- or B’ - component), "extensive" defects linked to nitrogen, the nitrogen content can be determined by computational decomposition of the absorption spectra.
To 2. If a diamond is coloured, it must absorb in the visible spectral range at wavelengths between 400 nm (violet) and 700 nm (red). The responsible for this defects we call colour centres, which also can cause absorptions in the adjacent Near Infrared and Ultraviolet. For their analysis the gemmologically often still used hand spectrometers are not sufficient, but here are also needed modern high resolution UV – VIS – NIR – absorption microscope spectrometers, that cover at least the wave length region between λ = 250 – 1100 nm.
As colour centres in the visible region and the adjacent Ultraviolet and Near Infrared are possible in the main two kinds of defects, the intrinsic lattice defects (vacancies, interstitial sites) and the extrinsic defects, mostly linked with nitrogen atoms. At the graphic representation of an absorption spectrum plotted on the ordinate absorption intensities depend on the transmitted thickness and the defect density, for example expressed as quantity per unit volume or as ratio, therefore given as absorption units per cm. The absorptions plotted on the abscissa as small lines or broader bands show the spectroscopic wavelengths resp. the corresponding energies. Characteristic optical spectra of vacancies (symbol V) are for example for neutral monovacancies ( this are vacancies with four electrons) at a wavelength of λ = 741 nm. Negative monovacancies (vacancies with five electrons) have an absorption line at 394 nm. These absorptions can have in the visible a colour effect by their more plane ending resonances. Similar effect is valid for the already mentioned as infrared absorbing nitrogen defects singular nitrogen atoms (called C) with an absorption peak at 270 nm and a continuous increase of the absorption from the Blue into the Ultraviolet and for the nitrogen atom pairs (called A) with absorption lines at 303, 307 and 315 nm. The absorption intensities for this are proportional in UV and IR, because in both cases the concentration dependence is valid. These absorptions also explain the relative opacity of the Ia-diamonds for ultraviolet light.
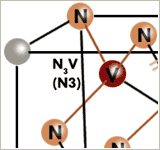 Much more distinctive concerning colouring and colour variations of the diamonds have the different couplings of nitrogen atoms and vacancies as colour centres, summarized as NV-centres, because their optical spectra are in the Visible region. A – aggregates (one pair of nitrogen atoms in place off two carbon atoms) with two coupled (neutral) vacancies (V2N2, symbolized as H3) have an absorption at 503 nm. B – Aggregates (four N – atoms tetrahedrically around a vacancy) connected with an additional vacancy (V2N2, H4) absorbs at 496 nm. Isolated nitrogen atoms with a neutral vacancy (NVo) resp. a negative charged vacancy (NV-) have their peaks at 575 nm resp. 637 nm. A number of further absorptions can be interpreted as colour centres, but there are some more, which can not yet be explained. Much more distinctive concerning colouring and colour variations of the diamonds have the different couplings of nitrogen atoms and vacancies as colour centres, summarized as NV-centres, because their optical spectra are in the Visible region. A – aggregates (one pair of nitrogen atoms in place off two carbon atoms) with two coupled (neutral) vacancies (V2N2, symbolized as H3) have an absorption at 503 nm. B – Aggregates (four N – atoms tetrahedrically around a vacancy) connected with an additional vacancy (V2N2, H4) absorbs at 496 nm. Isolated nitrogen atoms with a neutral vacancy (NVo) resp. a negative charged vacancy (NV-) have their peaks at 575 nm resp. 637 nm. A number of further absorptions can be interpreted as colour centres, but there are some more, which can not yet be explained.
The large spectra of colours, shades and colour saturations results then from the various proportions of the different colour centres. By irradiation, for example by high energy electrons, by heating, for example up to more than 800 °C in inert environment, or by high pressure – high temperature treatment, for example at pressures of 5 – 6 GPa and temperatures of 1500 – 2000 °C. colour centres, can heal, that colours can disappear, fade or change, or colour centres can generate, which can cause intensive colours, as for example the Fancy Colours, and colour changes. As well the IR – spectroscopic as the UV – Vis – spectroscopic methods of investigation are important at answering the questions, if and how a diamond had been treated.
|


